Affinity membrane absorbers for the capturing of virus particles
Membrane processes had been used for bioprocesses long before modern membrane industry has emerged. Their increasing application in chromatography is mainly triggered by the fact that they are not compressible compared to conventional bead supports and eliminate diffusion limitations. Over the last decades, new membrane systems have been developed to meet the requirements of the pharmaceutical industry to purify cell culture-derived viral particles and viral vectors. One focus is the application of highly specific affinity membrane adsorbers. For these systems economic capturing of large biomolecules is mainly limited by the properties of the affinity ligands. Particular attention has to be paid to the ligand interaction kinetics and the desorption conditions. Naturally, availability, stability, applicability in pharmaceutical production processes and ligand costs are also crucial points to be considered in the development of economic capturing processes.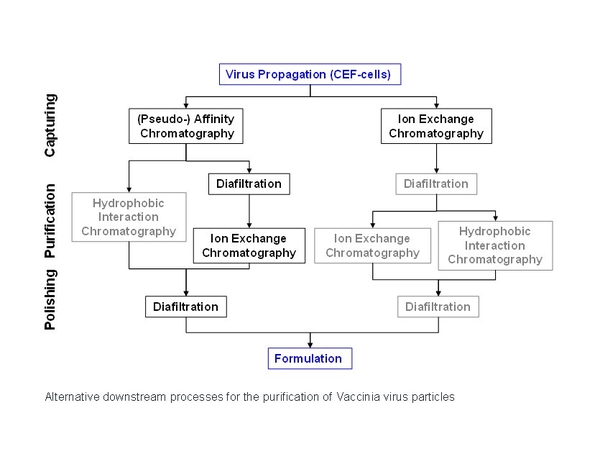
Our studies focus on applications of membrane adsorption chromatography to capture cell culture-derived influenza and vaccinia virus particles and compares these methods to bead based resins.
Madin-Darby canine kidney (MDCK) cell culture-derived influenza virus particles have been captured by anion exchange and affinity membrane adsorbers. The applied affinity and pseudo-affinity ligands were lectins, sulfated carbohydrates (e.g. heparin, sulfated glucose polymers) and specific peptides. The cost effectiveness of the process is heavily influenced by the selection of the specific, but in the case of influenza virus particles, substrain independent ligands. One possibility are peptide ligands e.g. against the sialic acid binding pocket of hemagglutinin, the major immunogenic envelope protein of influenza viruses. Identification of such ligands from synthetic libraries is the focus of current experiments.
The pseudo-affinity ligands employed for the capturing of primary chicken embryo fibroblast (CEF) culture-produced Modified Vaccinia Ankara (MVA) virus were sulfated carbohydrates. Here, in particular we compared the commercially available bead based resin Cellufine® sulfate with sulfated reinforced cellulose membranes. Furthermore, we compared the noticeable DNA depletion capabilities of this technology with classical ion exchange adsorbers.
Capturing both influenza and vaccinia virus particles the ligand specificity of affinity membrane adsorbers afforded a remarkable high degree of contaminant depletion. Up to 90% of the host cell DNA was eliminated compared to the starting content. Host cell proteins could be reduced to less than 5% of the initial amount, whereas an overall product yield of 80 to 90% could be achieved. In some applications host cell DNA and protein concentrations are close to meet regulatory requirements for vaccine products after a single unit operation. Hence, affinity membrane adsorption of cell culture-derived viral particles enables the capturing of viral particles at a high purity by a high volumetric throughput representing an economic tool for vaccine production processes.
Part of this project is done in close collaboration with Sartorius Stedim Biotech GmbH, Goettingen, Germany.
References:
1. Wolff, M.W.; Siewert, C.; Hansen, S.P.; Faber R.; Reichl, U.
Purification of cell culture-derived modified vaccinia ankara virus by pseudo-affinity membrane adsorbers and hydrophobic interaction chromatography.
Biotechnol. Bioeng., 107(2), 312-320 (2010)
2. Wolff, M.W.; Siewert, C.; Lehmann, S.; Post Hansen, S.; Djurup, R.; Faber, R.; Reichl, U.
Capturing of cell culture-derived modified Vaccinia Ankara virus by ion exchange and pseudo-affinity membrane adsorbers
Biotechnol. Bioeng., 105(4), 761-769 (2010)
3. Opitz, L.; Lehmann, S.; Reichl, U.; Wolff, M.W.
Sulfated membrane adsorbers for economic pseudo-affinity capture of influenza virus particles
Biotechnol. Bioeng. (2009), 103(6): 1144-1154
4. Opitz, L.; Zimmermann, A.; Lehmann, S.; Genzel, Y.; Lübben, H.; Reichl, U.; Wolff, M.W.
Capture of cell culture-derived influenza virus by lectins: Strain independent, but host cell dependent
J. Virol. Methods (2008) 154(1-2): 61-68
5. Opitz, L.; Salaklang, J.; Büttner, H.; Reichl, U. and Wolff, M.W.
Lectin-affinity chromatography for downstream processing of MDCK cell culture derived human influenza A viruses
Vaccine (2007) 25: 939-947




